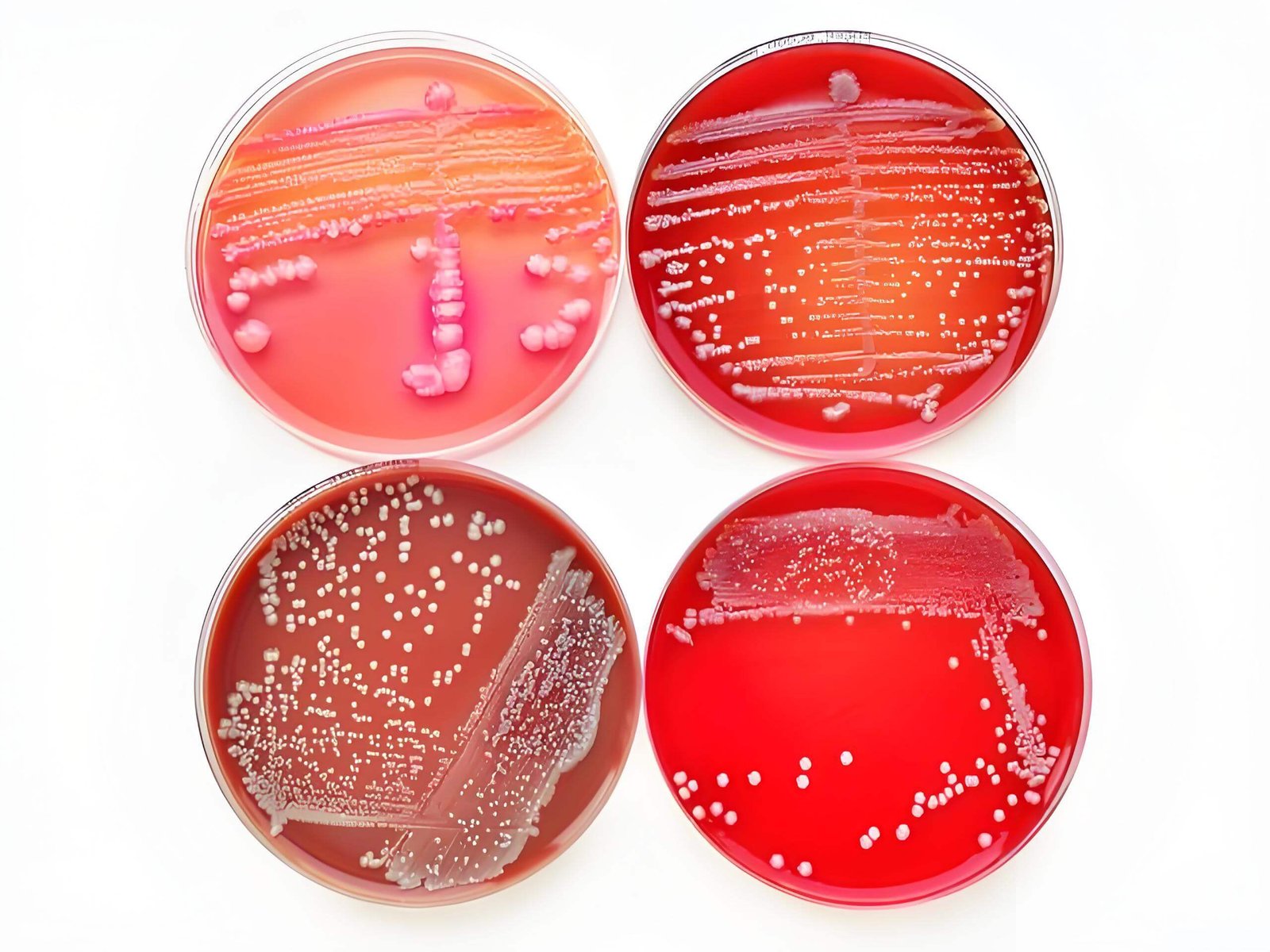
Entering the European cosmetics market requires navigating some of the world’s most rigorous safety regulations. At the heart of demonstrating product safety lies effective microbial testing, a process fundamentally dependent on the quality and compliance of your culture media. This guide explores how the right culture media is your key to meeting these strict European safety standards.
Understanding the Regulatory Landscape: EP and Cosmetics Regulation
The European Pharmacopoeia (EP) provides the legally binding standards for the quality of medicines and their components, which are directly referenced for cosmetic product safety. Compliance with EP methods, including the use of specified culture media, is not just a best practice—it’s a mandatory requirement for market access. Your choice of culture media must be precise, traceable, and validated to these standards to ensure your cosmetic microbial testing is recognized by European authorities.
Why Compliant Culture Media is Non-Negotiable
Using non-compliant or sub-standard culture media introduces significant risk. Inaccurate results can lead to:
- False Negatives: Allowing contaminated products to reach consumers, causing health issues and severe reputational damage.
- Regulatory Rejection: Your product documentation may be deemed insufficient, blocking your entry into the European market.
- Costly Recalls: Failure to identify contamination can result in expensive product recalls and legal repercussions.
Precise, pharmacopeia-compliant media is the first and most crucial line of defense in your quality control process, ensuring the accuracy and reliability of your data.
Building a Compliant Testing Workflow with Babio
A robust testing protocol extends beyond the culture medium itself. At Babio, we offer integrated solutions designed to support your compliance journey from start to finish.
- Pharmacopeia Medium Series: Our core range, including media fully compliant with the European Pharmacopoeia (EP), ensures your test methods meet the exact specifications required by regulators.
- Cosmetic Detection Series: These specialized media are formulated for the specific enumeration and detection of microorganisms in cosmetic products, aligning with the demands of the cosmetics regulation.
- Sterile Sampling Bags & Transport Media: Protect sample integrity from the moment of collection, ensuring that your results are a true reflection of your product’s quality.
- Ready-to-Use Culture Media & Petri Dishes: Minimize preparation errors and save valuable laboratory time while guaranteeing sterility and consistency in every test.
Conclusion: Trust Your Compliance to Specialized Solutions
Navigating European safety standards can be complex, but the foundation of your success is clear: reliable, compliant culture media. By partnering with a supplier who prioritizes regulatory alignment, you secure your product’s safety and your brand’s access to a key global market.
Ensure your cosmetics meet every requirement. Explore Babio’s Pharmacopeia Medium Series and complementary products to build a seamless, compliant quality assurance system.
Choose Babio for confidence in compliance.
Recent Posts
- Essential Tips for Proper Culture Media Storage and Handling
- Understanding the Role of Transport Culture Media in Clinical Diagnostics
- Breaking Down the Role of Culture Media in Pharmaceutical Research
- A Guide to Using Culture Media for Strict European Cosmetic Safety Compliance
- The Role of Culture Media in Ensuring Accurate Cosmetic Microbial Testing



