Description
Modified Cary-Blair Transport Media for Clinical Specimens
Modified Cary-Blair Transport Media is an essential tool for the collection and shipment of clinical specimens, particularly in diagnosing enteric infections. This media maintains the viability of enteric pathogenic bacteria during transit, making it crucial for accurate microbiological analysis. Enteric infections can arise from various bacteria, including Salmonella spp., Shigella spp., and Campylobacter spp.. Thus, using this transport media ensures that laboratories can effectively detect these pathogens.
Key Features and Principles
The design of Modified Cary-Blair Transport Media focuses on preserving specimens in a non-nutritive state. It does not contain any nutritional components, which allows for the long-term preservation of bacteria without promoting growth. The inclusion of sodium thioglycollate creates a low oxidation-reduction potential environment, crucial for maintaining bacterial viability. Additionally, disodium hydrogen phosphate acts as a buffer, while sodium chloride regulates osmotic pressure and bacterial cell membrane permeability.
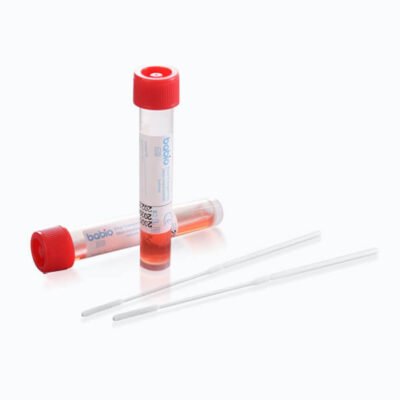
This transport media is available in 2 mL and 3 mL polypropylene screw-cap tubes, with packaging options of 20, 50, or 100 pieces per pack. Such flexibility allows healthcare facilities to choose quantities that best suit their needs.
Storage and Handling Guidelines
Modified Cary-Blair Transport Media is ready for immediate use and requires no preparation. Store the product sealed at temperatures between 2-25°C for up to 18 months. Avoid overheating, incubation, or freezing, as improper storage can compromise its efficacy. Always check for signs of damage or contamination before use, and do not use it if the expiration date has passed.
For optimal organism viability, transport specimens collected in this media directly to the laboratory within 2 hours. If immediate processing is not possible, store the specimens at 2-8°C and process them within 24 hours. Compliance with local regulations for specimen handling is essential to ensure safety and accuracy.
Quality Control and Limitations
Quality control procedures ensure that Modified Cary-Blair Transport Media is non-toxic to enteric pathogenic bacteria. Each batch is tested for pH stability and its ability to maintain viable bacteria at room temperature. If any quality control issues arise, patient results should not be reported.
However, it’s important to recognize the limitations of this media. The condition, timing, and volume of the specimen play a significant role in obtaining reliable culture results. Follow recommended guidelines for specimen collection closely. This product is designed to be used exclusively with compatible swabs; using tubes or swabs from other sources may affect performance.
Performance and Application
In an aseptic environment, collect samples of Vibrio parahaemolyticus, Salmonella enterica, and Shigella flexneri using a swab. Store these samples at 20-25°C for 48 hours before transferring them to blood agar medium. Incubate at 36±1°C for 18-24 hours to observe bacterial viability. The bacteria should grow well, confirming the effectiveness of the transport media.
In summary, Modified Cary-Blair Transport Media is critical for the safe collection and transport of clinical specimens. By preserving the viability of enteric pathogens, this media enables accurate diagnosis and treatment of enteric infections. For laboratories seeking reliable transport solutions, this media is an indispensable component of microbiological testing. visit our [Transport culture medium] for more related products



-1-400x400.jpg)
Reviews
There are no reviews yet.